Pediatric DTG FAQs
- hivtoolkit
- Oct 11, 2021
- 1 min read
Updated: Jan 13, 2022

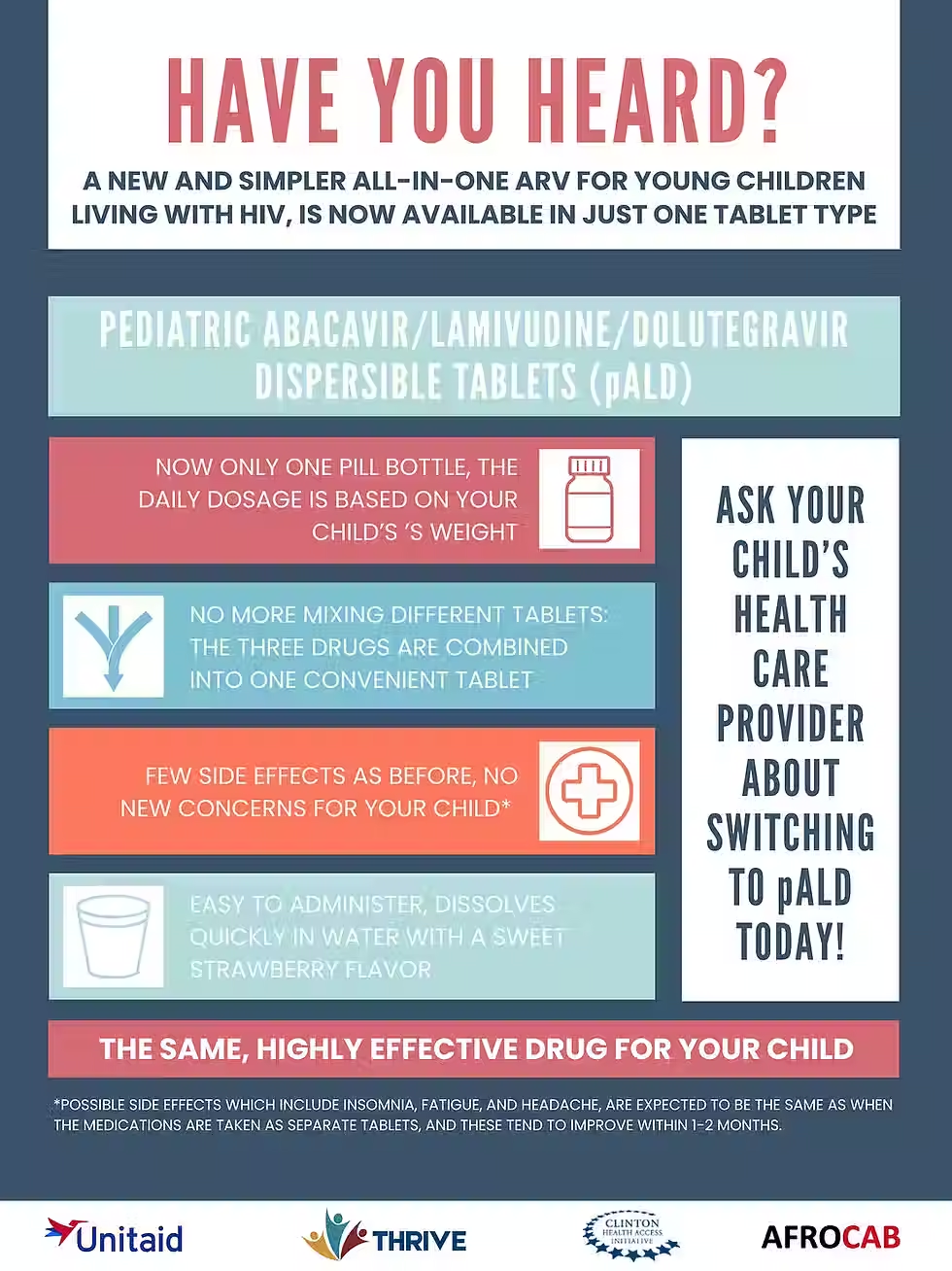
This document is meant to assist national HIV programs in pDTG adoption. This document provides a summary of pDTG, including advantages over other ARVs, cost, dosing, administration, side effects, and transition considerations.


Comments