GAP-f pDTG Implementation Considerations for National Programmes
- hivtoolkit
- May 24, 2022
- 2 min read

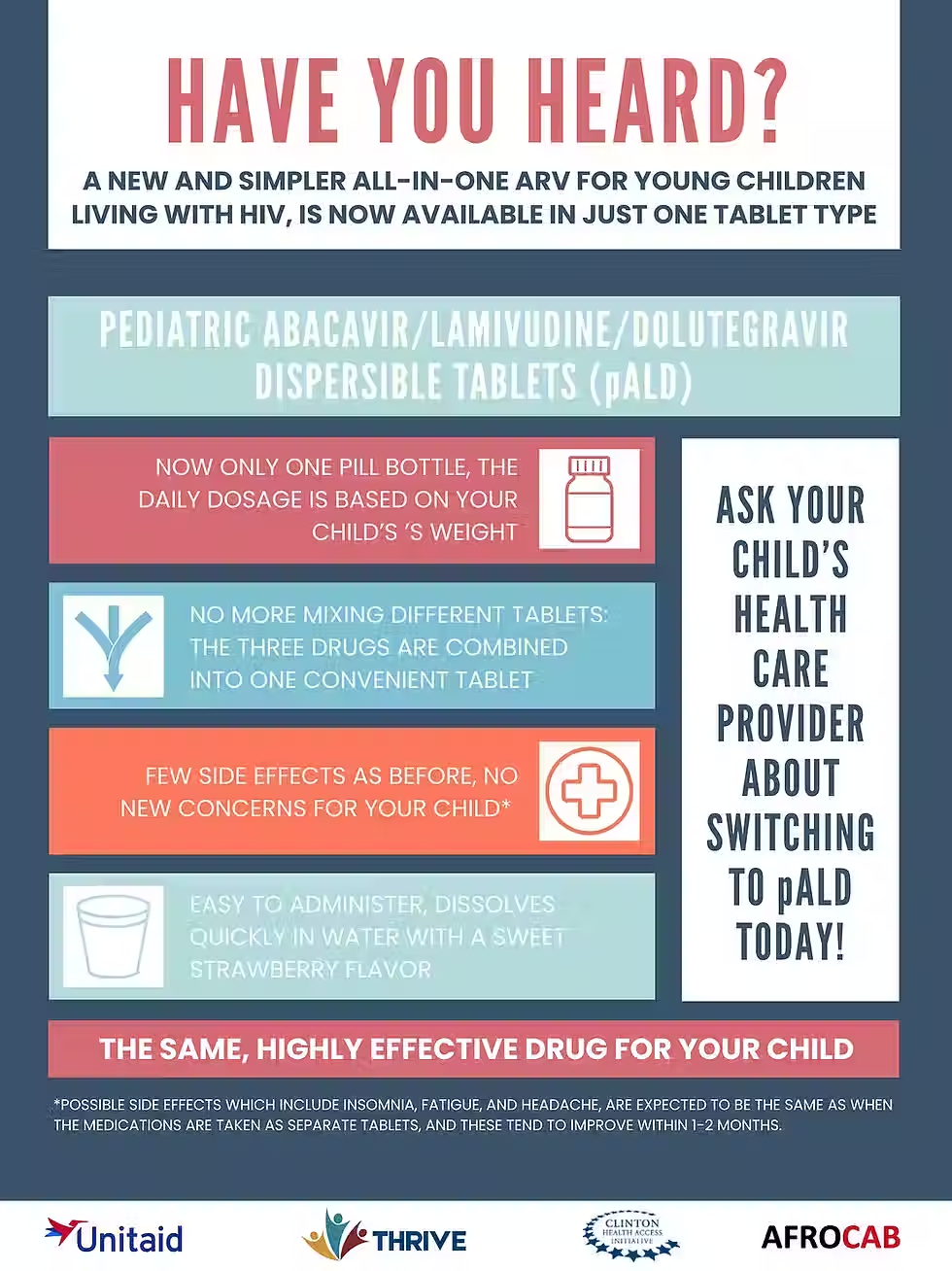
In mid-2021, national HIV programmes began to transition treatment for children living with HIV to dolutegravir (DTG) 10 mg scored, dispersible tablets, also known as paediatric DTG (pDTG). Rapid introduction and rollout of pDTG is a priority to implement the World Health Organization (WHO) guidelines and ensure that children living with HIV receive the best available first- and second-line HIV treatment as soon as possible.
To ensure children are transitioned to pDTG safely and effectively, the GAP-f pDTG Task Team developed some implementation considerations for national HIV programmes, implementing partners, and service providers.

About the GAP-f pDTG Task Team: The GAP-f pDTG Task Team is a forum for coordination among partners involved in introduction of pDTG scored, dispersible tablets. The pDTG Task Team is a platform to share what partners are already doing, identify where work can complement each other and most importantly identify the gaps that need to be addressed and where GAP-f could help ensure that paediatric DTG can be scale-up as quickly as possible. Organizations participating in the pDTG Task Team include: Clinton Health Access Initiative (CHAI), Drugs for Neglected Diseases initiative (DNDi), Elizabeth Glaser Pediatric AIDS Foundation (EGPAF), Global Fund to Fight AIDS, Tuberculosis and Malaria, International AIDS Society (IAS), International Center for AIDS Care and Treatment Programs (ICAP), Médecins Sans Frontières (MSF), Medicines Patent Pool (MPP), Paediatric-Adolescent Treatment Africa (PATA), UNAIDS, UNICEF, US Agency for International Development (USAID), US Centers for Disease Control and Prevention (US CDC), US Department of State, World Health Organization (WHO). For more info, visit https://www.who.int/initiatives/gap-f.


Comments