GAP-f pALD Introduction and Rollout Planning Considerations for National Programmes
- hivtoolkit
- May 24, 2023
- 1 min read
Updated: Jul 22, 2023

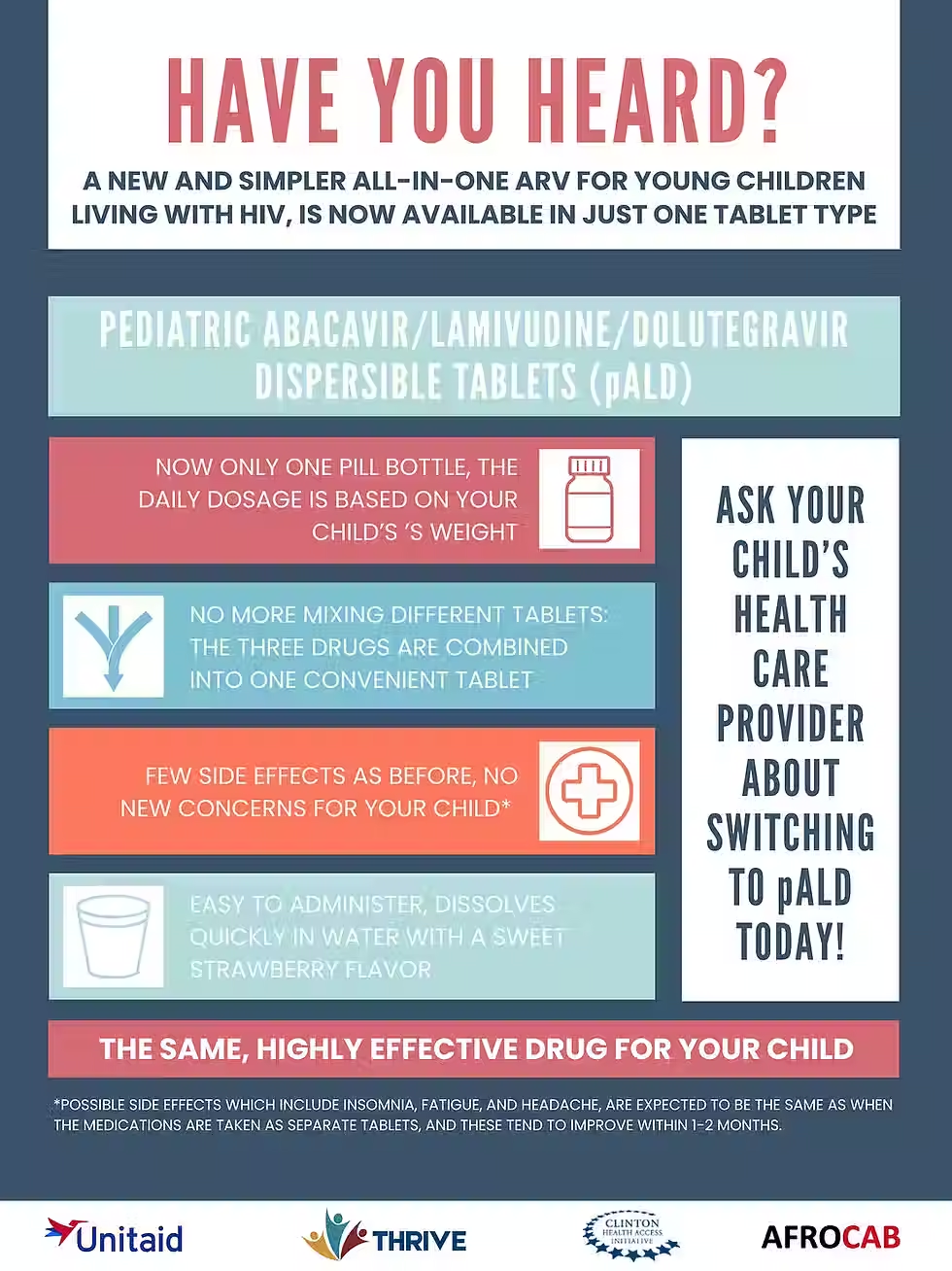
The GAP-f pDTG task team has developed a brief outlining product introduction and rollout considerations for national programmes when introducing the fixed-dose combination (FDC) of paediatric abacavir/lamivudine/ dolutegravir (pALD). Specifically, this brief aims to inform the transition from pDTG + pABC/3TC to pALD.
The brief is available in English, Swahili, French, and Spanish, and Portuguese.

About GAP-f: GAP-f is a WHO Network hosted within the Research for Health Department in the Science Division at WHO and was created to respond to the paediatric treatment gap. Following the resolution at the 69th World Health Assembly on promoting innovation and access to quality, safe, efficacious, and affordable medicines for children, GAP-f was conceived to build on and formalize the model developed within the HIV community to provide a sustainable mechanism that ensures that safer, more effective, and more durable paediatric formulations are developed and made available to children against an accelerated timeline. More information is available at https://www.who.int/initiatives/gap-f.


Comments